Overview of global RNA drug research and development
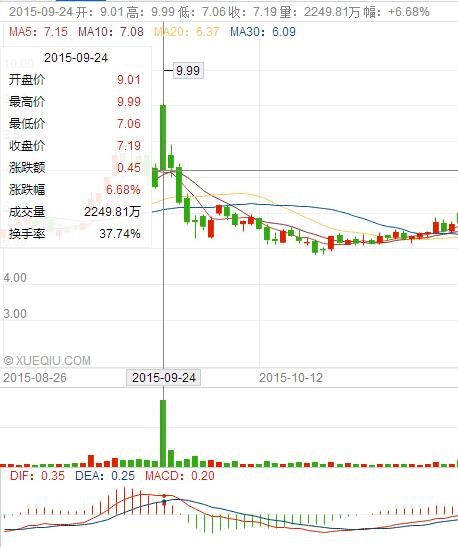
Last week, Arrowhead's stock price rose for several days because of the positive data of ARC-520 clinical phase IIb, a candidate drug for hepatitis B, released by Arrowhead at the annual meeting of the European Society of Hepatology in 2016. In fact, it is similar to the clinical phase IIa data released on September 24, 2015, and the capital market reaction is similar.
According to the most basic central principle, biological information from DNA to RNA, then to protein, and finally to protein to perform biological functions. Current drugs are basically acting on proteins (such as enzymes, GPCR, RTK, ion channels), regulating biological pathways at the protein level. RNA drugs regulate biological pathways at the upstream RNA level at the protein level to achieve therapeutic purposes.
At present, there are four main types of RNA drugs: siRNA, ASO, nucleic acid aptamers, and microRNAs.
The mechanism of action is different as follows:
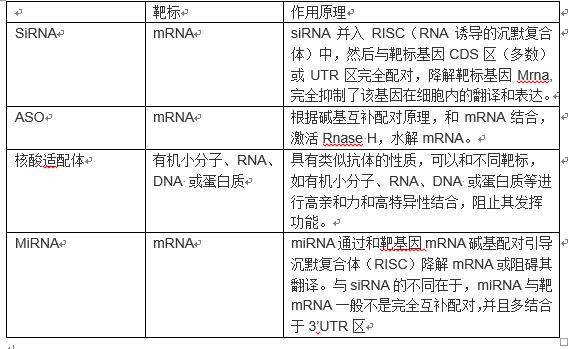
SiRNA drugs are the most popular type of RNA drugs nowadays. Since Andrew Fire and Craig Mello first revealed the phenomenon of RNAi in online worms in 1998, RNAi research has been extremely heated, and then companies began to develop siRNA drugs. In 2004, Opko Company developed Bevasiranib, a siRNA drug for the treatment of wet age-related macular degeneration. It was the first clinical trial related to siRNA. Since then, siRNA has attracted wide attention as a gene therapy drug. Thereafter, the research and development of siRNA drugs has entered a crazy stage: pharmaceutical giants Roche, Merck, Pfizer, Sanofi, Abbott and so on entered this field. Andrew Fire and Craig Mello found that after winning the 2006 Nobel Prize, they inspired a number of companies to enter the field, and Opko's Bevasiranib was also expected to do so. However, with the deepening of the research, the giants gradually found that siRNA is extremely difficult to prepare, either ineffective or can produce serious autoimmune response. OPKO also announced on March 6, 2009 that Bevasiranib was terminated for phase III clinical trials of wet-AMD. Immediately, siRNA drug research and development entered a low ebb. In 2010, Roche Pharmaceuticals, which has invested nearly $500 million in research and development of RNAi technology, terminated internal research and development of RNAi. In 2011, Pfizer and Abbott withdrew from RNAi therapy, and Merck shut down the RNAi lab they received from Sirna Therapeutics for $1.1 billion in 2006.
Over the years, with the continuous improvement of siRNA delivery technology, the temperature has gradually increased. At present, siRNA drugs are concentrated in many of the best companies in the field of RNA drugs, such as Alnylam, Tekmira and so on. At present, most of the design schemes adopt tuschl rule (19nt + dtdt):
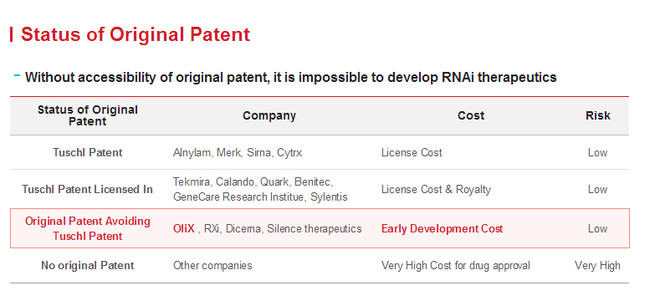
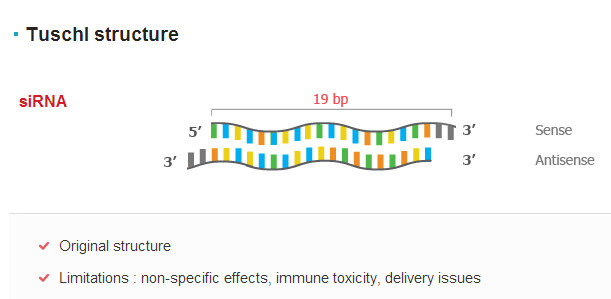
Pictures from: OliX Pharmaceuticals, Inc. SiRNA drug core technology in addition to design methods, because siRNA is easily degraded by nuclease after intravenous injection, high kidney clearance rate, poor cell uptake efficiency, clinical application is limited, so the selection of safe and stable transporter to deliver to target tissue cells can play a pharmacodynamic effect. Generally, siRNA transporters are divided into viral and non-viral vectors: viral vectors have high transfection efficiency, but can cause immune response; non-viral vectors have a wide range of sources, controllable chemical structure and are easy to be prepared in large quantities. At present, the non-viral vectors and their general structures adopted by companies are mainly as follows:
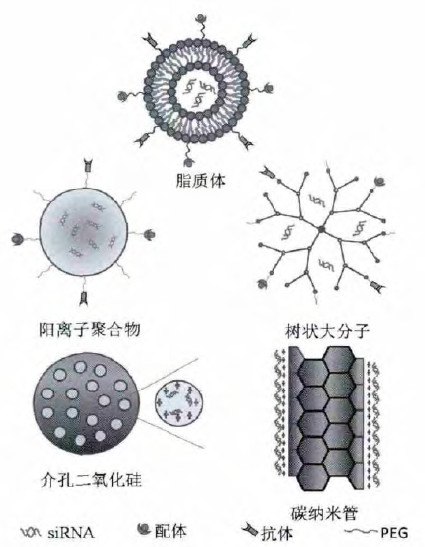
Pictures from: CNKI
Corporate Delivery Platform:
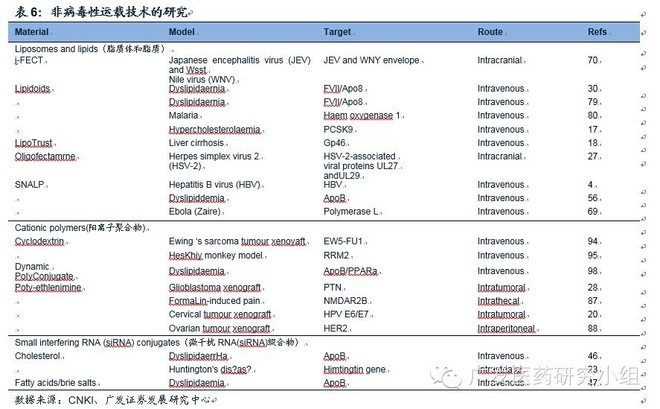
Research report from Guangfa Securities at the beginning of last year: Small Nucleic Acid Market
Although there are some obvious mistakes in the middle, we can look for them and find out that the whole is OK.
Alnylam R&D pipeline and progress: http://www.alnylam.com/product-pipeline/
Other companies can go to the official website to see the R&D pipeline and progress:
Arrowhead
Benitec Biopharma
Callmmune
Dicerna
Gradalis (shRNA)
Quark
RXi
Senesco
Silence Therapeutics
Silenseed
Tekmira
The second type is ASO (antisense oligonucleotide, also referred to as ASON, AON), or antisense oligonucleotide. ISIS Pharmaceuticals (renamed Ionis after the terrorist organization) is the absolute hegemony in this field. The first antisense drug, Vitravene (Fomivirsen), was approved by the FDA in 1998. This is thiophosphate DNA, which is used to treat retinitis in AIDS patients caused by Cytomegalovirus. But its usefulness was so scarce that it brought in only $157,000 in sales for ISIS Pharmaceutical and Novartis in 2001, and then dropped out of the market.
In 2013, ISIS Pharmaceutical and Genzyme launched the second antisense oligonucleotide of Kynamro (Mipomersen) for the treatment of a rare homozygous family hyperlipidemia (HoFH) as a lipid-lowering drug and dietary supplement to reduce LDL cholesterol, apolipoprotein B, total cholesterol and LDL cholesterol.
Mipomersen sodium is a synthesized sodium salt of phosphorothioate oligonucleotide with 20 nucleotide RNA and DNA heterozygous chains. Its sequence is 5'-GCCUCAGTCTGCTTCGCACC-3'.
The specific structure information can be queried on drugbank.
Another antisense oligonucleotide drug already on the market is Alicaforsen of ISIS Pharmaceuticals. Alicaforsen is an antisense oligonucleotide of ICAM-1 for the treatment of chronic enteritis and pouch colitis.
Ionis R&D pipeline: http://www.ionispharma.com/pipeline/
Other antisense oligonucleotide drugs include Aganirsen (IRS1 inhibitor, Gene Signal), Belagen pumatucel-L (TGFB2 inhibitor, NovaRx), Drisapersen (DMD regulator, GSK), Custirsen (CLU inhibitor, OncoGenex), Kappaproct (NFKB1 inhibitor, InDex), Trabedersen (TNFSF13 inhibitor, Antisense Pharma).
Nucleic acid aptamers:
Nucleic acid adapters and antibodies are similar in nature. The first to be listed is Pegaptanib (Eyetech and Pfizer) approved by the FDA in December 2004, which is an oligonucleotide (RNA Aptamer) of the monomer of VEGF-A165 for "wet" AMD.
There is also an antithrombotic nucleic acid aptamer ARC183, the antineoplastic drug AS1411 (British Antisoma Pharmaceuticals)
MiRNA drugs:
MiRNA is a kind of ncRNA which has been studied very hot in recent years. With the development of research, many drugs have entered the clinic. Like siRNA drugs, the most important is delivery platform. Leading companies, such as Miagen, are already planning to go public.


